Abstract
Von Willebrand factor (VWF) is a large multimeric glycoprotein of critical importance for both primary and secondary hemostasis due to its role in platelet activation and stabilization of coagulation factor VIII (FVIII), respectively. Mutations affecting VWF synthesis, secretion, or function result in one of 6 distinct subtypes of von Willebrand disease (VWD) with mutations inhibiting FVIII binding classified as type 2N. Due to low circulating levels of plasma FVIII, type 2N patients can be misdiagnosed with hemophilia A. Because the clearance of FVIII is dominated by its association with VWF, and given recent evidence demonstrating an immuno-protective role of VWF for FVIII, understanding the molecular interaction between FVIII and VWF remains critically important for both hemophilia A and type 2N VWD therapeutic development. However, limited structural information remains a major limitation for rational design studies. Recently, ancestral sequence reconstruction (ASR) was performed to infer the sequences of FVIII predicted to have existed throughout mammalian evolution. ASR provides a platform for high-resolution mapping of sequential differences within a phylogeny while maintaining a high probability of retaining function. Sequential changes in the amino acid sequence of FVIII appears to have resulted in altered biochemical properties including biosynthesis and stability. In this study, we demonstrate that a previously characterized ancestral (An) FVIII molecule with 95% identity to human FVIII, termed An53-FVIII, exhibits a 2.5 to 5-fold increase in binding to human VWF over commercial recombinant FVIII products, suggesting a dynamic association throughout evolution. We sought to further explore the FVIII-VWF interaction through ASR of VWF and biochemical analysis.
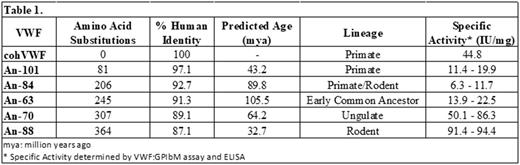
A phylogenetic tree was constructed for VWF and the ancestral amino acid sequences predicted through Bayesian inference. A compiled list of 59 extant sequences allowed accurate prediction of the sequences extending beyond the class Mammalia. Of the resulting 50, 5 An-VWF sequences representing significant branch points in primate, rodent, and ungulate evolution were selected for synthesis de novo and subsequent biochemical characterization. All sequences, including an additional human (h) VWF cDNA, were human codon optimized (co). These sequences are predicted to span 105 million years of evolution and contain 81 to 364 amino acid substitutions (Table 1). Heterologous expression in HEK293 cells demonstrated that all An-VWF sequences resulted in secreted antigen as determined by ELISA using anti-human VWF polyclonal antibodies. Furthermore, immunohistochemistry of HEK293 cells expressing An-VWF revealed the formation of pseudo Weibel-Palade bodies by all VWF proteins. Stably transected, clonal producer cell lines were generated and utilized for recombinant VWF production and purification using heparin-sepharose affinity chromatography. Consistent with our IHC studies, multimer analysis of the purified proteins revealed the presence of high molecular weight multimers equivalent to human plasma VWF. To test the functionality of An-VWF, the VWF:GPIbM assay was performed. Compared to an observed specific activity of 45 IU/mg VWF for cohVWF, An-VWF molecules displayed specific activities ranging from 6 to 94 IU/mg. These data suggest that VWF and GPIb may have co-evolved to regulate platelet adhesion and activation. Hydrodynamic injection of An63-, An88-, and An101-VWF plasmid DNA into VWF deficient mice revealed plasma VWF levels of 1.44 ± 0.48, 0.72 ± 0.16, and 2.9 μg/mL at 24 hours after administration, respectively (n = 4, 3, and 1). Additionally, endogenous mouse (m) FVIII levels in these mice were elevated to 61 ± 9, 75 ± 16, and 67% of normal levels from a basal 12.9 ± 2.4%. This data demonstrates that liver synthesized An-VWF retains FVIII association and stabilization. Molar ratios of An88-VWF:mFVIII are 6:1 compared to 14:1 and 25:1 for An63- and An101-VWF, respectively, supporting the coevolution hypothesis for VWF and FVIII. Cumulatively, this study further validates the utility of ASR for the generation of functional sequences for complex proteins with a high propensity for inactivating mutations. Further analysis of ancestral sequences may provide greater resolution of amino acid sequences responsible for biochemical properties including clearance and FVIII association.
Gaucher: General Genomics: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal